DEEPVESSEL FFR is Approved for Clinical Use in China
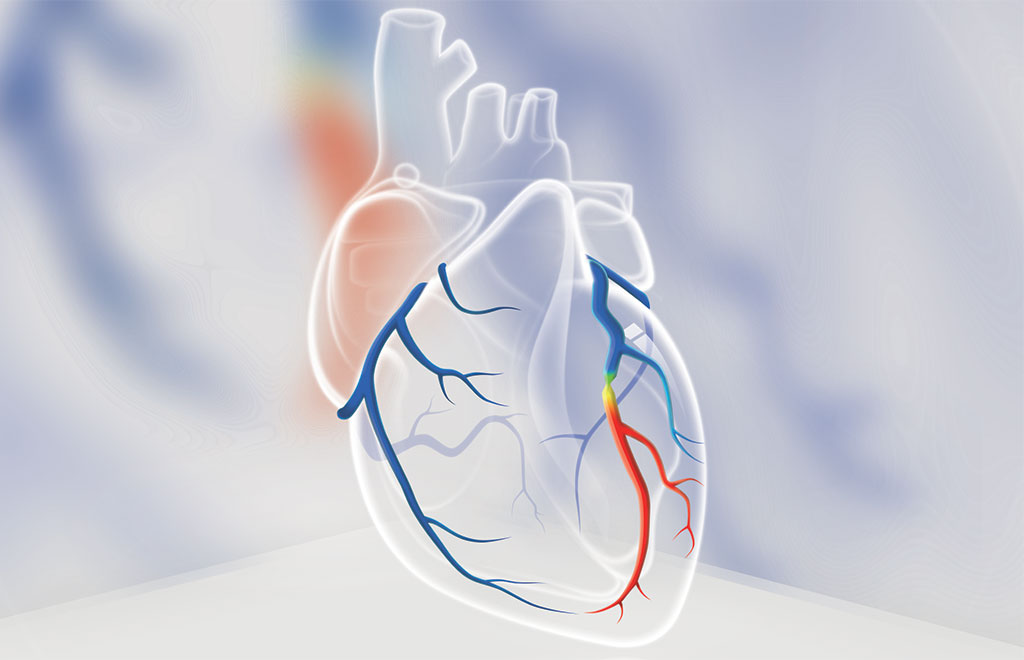
DEEPVESSEL FFR, an innovative CT-FFR technology, uses deep learning to perform a physiological functional assessment of the coronary arteries in minutes from coronary CT angiography scans. DEEPVESSEL FFR test can non-invasively identify ischemia in patients with suspected coronary artery disease before sending patients for further invasive coronary angiography.
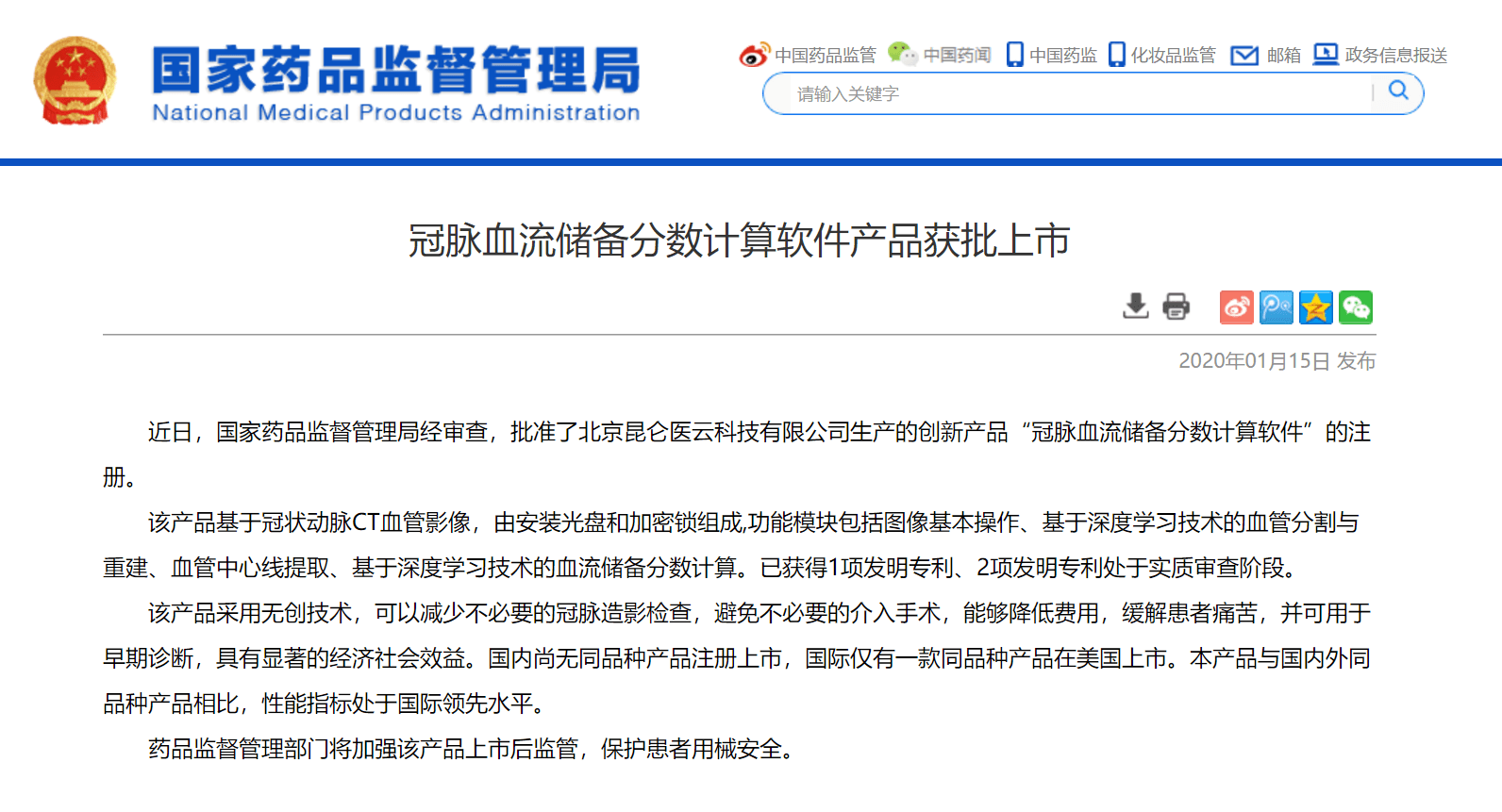
On January 15, 2020, the National Medical Products Administration (NMPA) in China issued the registration certificate approving DEEPVESSEL FFR for clinical use. This certificate allows Keya Medical to offer DEEPVESSEL FFR service to millions of patients with stable angina in China. DEEPVESSEL FFR is the only approved device for use in China for computing CT-FFR from coronary CT angiograms. The precise diagnostic information provided by noninvasive DEEPVESSEL FFR can support cardiologists in making better treatment decisions, and help patients avoid discomfort and potential complications from unnecessary invasive examinations and surgical procedures.
DEEPVESSEL FFR is the world’s first licensed noninvasive CT-FFR product based on deep neural networks. It was the first artificial intelligence (AI) product to enter the NMPA’s Innovative Medical Device program, and the first AI product approved for marketing by NMPA. At present, no other equivalent product is registered for use in China. Worldwide, there is only one other similar product in clinical use. The clinical performance of DEEPVESSEL FFR compares favorably with published reports of the other similar product.

Recent Comments