
Keya Medical
AI for Smarter Healthcare
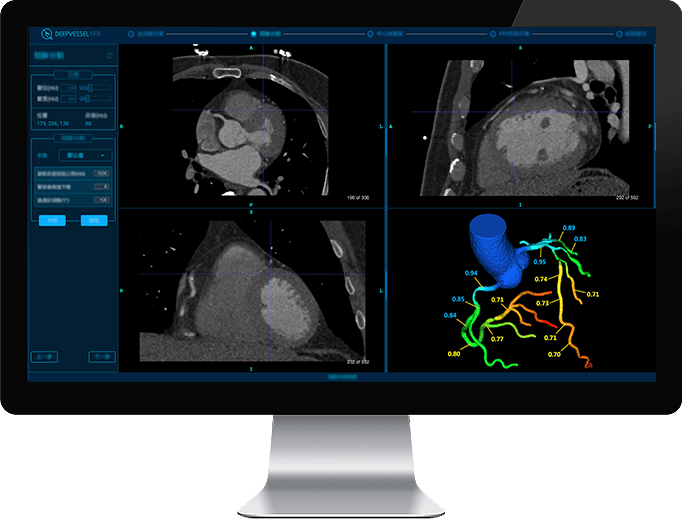
CT FFR Analysis
Enhancing Diagnosis and Management for Coronary Artery Disease Patients
DEEPVESSEL FFR is a AI-enabled, non-invasive CT fractional flow reserve (FFRCT) analysis. It is an augmentative physiological functional assessment of the coronary arteries using coronary computer tomography angiograms. The software can reduce unnecessary invasive testing, improve patient safety, and lower the cost of cardiovascular testing.
A New Category I CPT code for non-invasive FFR
Category I CPT Code
Starting January 1, 2024, medical practices will implement a new category I CPT code (75580) for non-invasive FFR estimates derived from coronary CT angiography software analysis.
The American College of Cardiology (ACC), American College of Radiology (ACR), and the Society of Cardiovascular Computed Tomography (SCCT) jointly proposed this change to the American Medical Association. It replaces four prior category III CPT codes.
Available in the USA, EMEA, China, Singapore
CE-Marked
2018
NMPA-Approved
2020
FDA-Cleared
2022
HSA-Certified
2023
Advantages
Precise
Comprehensive
Cost Saving
Physician Autonomy
Streamlined Workflow
Efficient
FFRCT and DVFFR have transformed my practice, allowing me to provide superior patient care and achieve better outcomes. I am sincerely grateful for this invaluable resource provided by Corazon Imaging.
News
AI-based FFRct: Conversation with Zwanger-Pesiri Radiology
This industry-sponsored episode of The Donut of Destiny podcast features a practical discussion on AI-based CT-derived fractional flow reserve (CT-FFR), highlighting how outpatient imaging centers are incorporating functional assessment alongside coronary CT angiography. Zwanger-Pesiri Radiology leaders discuss implementation, scalability, and clinical considerations in everyday practice.
The Future of AI and CCTA: Enhancing Decision-Making for Patient Management
Keya Medical’s webinar about the transformative role of AI and CCTA in optimizing patient management featuring Dr. John Rumberger and Dr. Asif Ali.
Sponsored webinar | Future of AI in CCTA: Enhancing decision-making for optimal patient management
Sponsored by Keya Medical webinar to spotlight AI and CCTA advancements in cardiovascular care.


Intended for the U.S. market only. Not for EU promotional use.



