The ADAPT Study: Assessment of the DiAgnostic Performance of DeepVessel FFR in SuspecTed Coronary Artery Disease
SUMMARY
DEEPVESSEL FFR is a medical device that is designed to extract three- dimensional coronary tree structures and generate computed tomography-derived fraction flow reserve (FFR) values from coronary CT angiogram images. The primary objective of this multi-center clinical validation study is to validate the clinical performance of DEEPVESSEL FFR in identifying patients with myocardial ischemia due to significant obstructive coronary artery diseases.
DETAILED DESCRIPTION
Coronary artery disease (CAD) is the most common type of heart disease, and it is the leading cause of death worldwide in both men and women. CAD happens when the coronary arteries become hardened and narrowed, which is due to the buildup of cholesterol-containing deposits-plaque on the inner vessel wall. As the plaque grows, less blood can flow through the arteries due to the vessel narrowing. Decreased blood flow can then lead to chest pain (angina), shortness of breath, or even a heart attack.
Fractional flow reserve (FFR), a measure of blood flow reduction caused by vessel narrowing, is accepted as gold standard for assessing the functional significance of stenotic lesions. Multiple randomized trials have demonstrated that FFR has excellent diagnostic value in identifying functionally significant lesions and guiding coronary revascularization procedures. However, FFR is measured invasively through a pressure wire-based cardiac catheter procedure in the catheterization lab. Current guidelines recommend assessing myocardial ischemia of stable patients with CAD through non-invasive functional testing before considering invasive coronary angiography (ICA) or conducting myocardial revascularization.
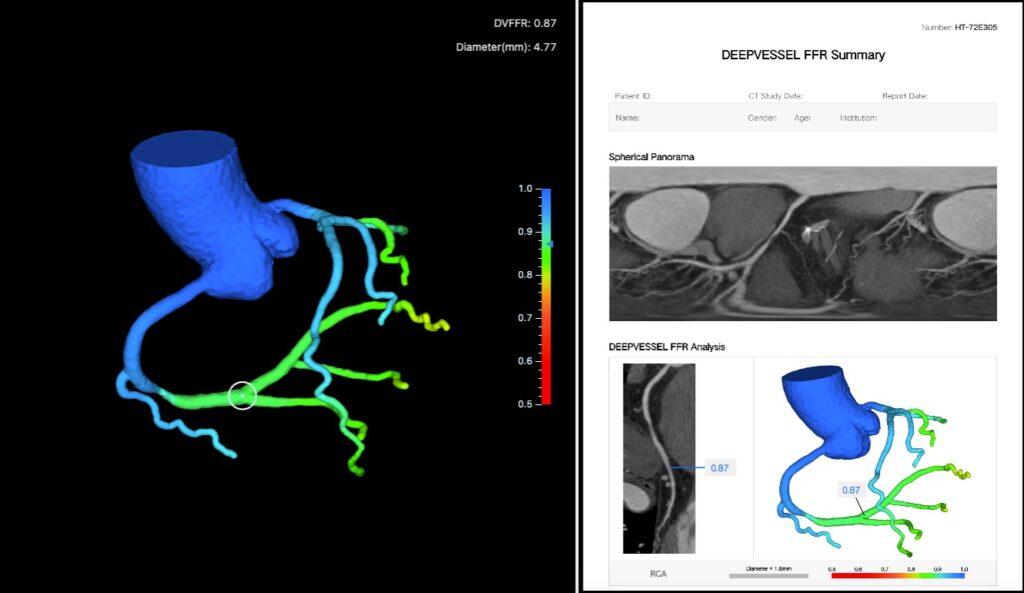
DEEPVESSEL FFR (DVFFR) is a software medical device that is designed to extract three- dimensional coronary tree structures and generate computed tomography -derived FFR values from coronary CT angiogram (CTA) images. It uses deep learning neural networks that encode imaging, structural, and functional characteristics of coronary arteries and learn complex mapping between FFR values and the encoded information. The quantitative FFR analysis based on the coronary CTA images can help clinicians assess the physiological function in patients with CAD non-invasively.
The primary objective of this study is to evaluate the diagnostic performance of DVFFR software in identifying patients with significant obstructive CAD causing myocardial ischemia, using invasively measured ICA FFR as the reference standard.
STUDY DESIGN
| Study Type: | Observational |
| Actual Enrollment: | 302 participants |
| Observational Model: | Case-Only |
| Time Perspective: | Retrospective |
| Actual Study Start Date: | August 10, 2020 |
| Actual Primary Completion Date: | December 1, 2021 |
| Actual Study Completion Date: | December 31, 2021 |
GROUPS AND COHORTS
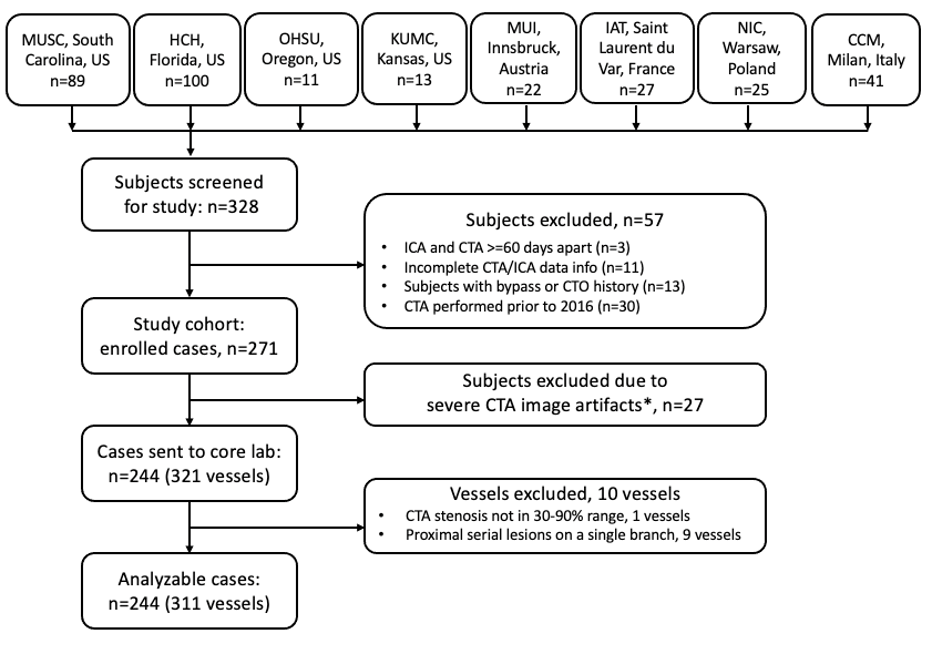
Patients with suspected CAD containing at least one 30%-90% coronary CTA stenosis.
Patients’ datasets with suspected CAD containing at least one 30%-90% coronary CTA stenosis; and ICA-FFR was measured on vessels with diameters greater than 2 mm will be analyzed. Diagnostic performance based on CT-derived FFR using DVFFR software will be compared with the diagnostic performance from ICA-FFR measurements.
OUTCOME MEASURES
Primary Outcome Measures
- Sensitivity of DVFFR at the vessel level in identifying ischemic lesions, i.e., DVFFR value≤80, from coronary CTA images, using ICA-FFR measurement as a reference standard. [ Time Frame: through study completion, an average of 1 year]
On the vessel level, if a vessel with at least one stenosis lesion with a FFR measurement less or equal to 0.80, this vessel is ischemic. A true positive on the vessel level is defined as a vessel containing at least one stenosis with DVFFR value ≤0.80 and its corresponding reference ICA-FFR value is also ≤0.80. - Specificity of DVFFR at the vessel level in identifying ischemic lesions, i.e., DVFFR value≤80, from coronary CTA images, using ICA-FFR measurement as a reference standard. [ Time Frame: through study completion, an average of 1 year]
On the vessel level, if a vessel with at least one stenosis lesion with a FFR measurement less or equal to 0.80, this vessel is ischemic. A true positive on the vessel level is defined as a vessel containing at least one stenosis with DVFFR value ≤0.80 and its corresponding reference ICA-FFR value is also ≤0.80.
Secondary Outcome Measures
- Diagnostic accuracy, positive predictive value (PPV) and negative predictive value (NPV) of DVFFR at the vessel level [ Time Frame: through study completion, an average of 1 year]
On the vessel lever, if a vessel with at least one stenosis lesion with a FFR measurement less or equal to 0.80, this vessel is ischemic. A true positive on the vessel level is defined as a vessel containing at least one stenosis with DVFFR value ≤80 and its corresponding reference ICA-FFR value is also ≤0.80.
- Diagnostic performance including sensitivity, specificity, accuracy, PPV and NPV of DVFFR at the patient level [ Time Frame: through study completion, an average of 1 year]
At the patient level, if a patient has at least one vessel that has been identified as causing ischemia, this patient is considered a patient positive for ischemia. A true positive on the patient level is defined as when a patient has at least one lesion with a DVFFR value ≤80 and its corresponding reference ICA-FFR is also ≤0.80.
- Per-vessel Pearson correlation coefficient between DVFFR and ICA-FFR values [ Time Frame: through study completion, an average of 1 year]
Correlation between CT-derived DVFFR values and wire-measured ICA-FFR values will be evaluated at the vessel level.
- Diagnostic performance (including sensitivity, specificity, accuracy, PPV and NPV) in detecting hemodynamically significant coronary obstruction using DVFFR and coronary CTA alone, on both vessel level and patient level. [ Time Frame: through study completion, an average of 1 year]
For coronary CTA, hemodynamically significant obstruction of a coronary artery is defined as a stenosis ≥50%. The per-patient stenosis degree will be specified as the most severe stenosis among the major epicardial artery vessels presented in coronary CTA.
- Stratified analyses on different subgroups of subjects’ data [ Time Frame: through study completion, an average of 1 year]
Stratified analyses on different subgroups of subjects’ data, ranging from patient demographics and disease conditions.
ELIGIBILITY CRITERIA
| Ages Eligible for Study: | 18 Years and older (Adult, Older Adult) |
| Sexes Eligible for Study: | All |
| Accepts Healthy Volunteers: | No |
| Sampling Method: | Probability Sample |
Study Population
Patients with suspected CAD containing at least one 30%-90% coronary CTA stenosis.
Inclusion Criteria
- Patients’ age ≥18 years.
- Has coronary CTA images acquired by ≥64 multidetector row CT scanner, no earlier than 2016 and within 60 days of the ICA-FFR procedure.
- Coronary CTA image shows at least one vessel segment (≥2mm diameter) with a diameter stenosis of 30%-90%.
Exclusion Criteria:
Patients with any of the following conditions at the time of CTA imaging:
- Acute myocardial infarction.
- Unstable angina.
- Pulmonary edema.
- Heart function classification level III and IV (NYHA heart function classification);
- Implantable cardioverter defibrillator (ICD).
- Prior percutaneous coronary intervention (PCI) or pacemaker surgery.
- Prior coronary artery bypass grafting (CABG) surgery.
- Prior heart valve replacement.
- Prior history of complex congenital heart disease.
- Prior history of cardiomyopathy.
- BMI >35.
- Coronary total occlusion.
LOCATIONS & CONTACTS
| Holy Cross Health | Fort Lauderdale, Florida, United States |
| University of Kansas Medical Center | Kansas City, Kansas, United States |
| Oregon Health & Science University | Portland, Oregon, United States |
| Medical University of South Carolina | Charleston, South Carolina, United States |
| Vanderbilt University Medical Center | Nashville, Tennessee, United States |
| Medical University Innsbruck | Innsbruck, Austria |
| Institute of Arnualt Tzanck | Nice, Saint-Laurent-du-Var, France |
| University of Ferrara | Ferrara, Italy |
| University of Milan | Milan, Italy |
| National Institute of Cardiology | Warsaw, Poland |
Sponsors and Collaborators: Keya Medical, Medical University of South Carolina
Investigators: Principal Investigator: Joseph Schoepf, MD, Medical University of South Carolina
STUDY RESULTS
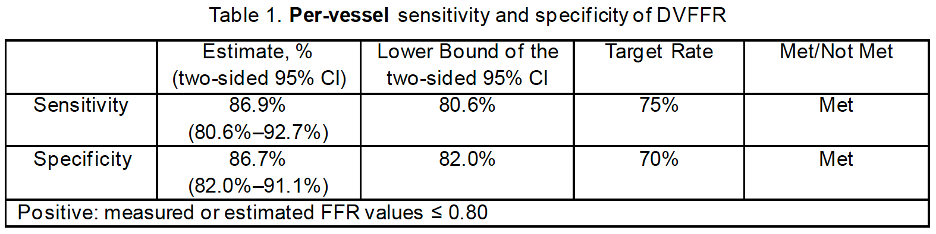
At the vessel level, the observed sensitivity of DVFFR was 86.9% with a two-sided lower bound 95% CI of 80.6%, and the observed specificity of DVFFR was 86.7% with a two-sided lower bound 95% CI of 82.0%. Both 95% CI lower bounds for sensitivity and specificity exceeded the performance target of 75% and 70%, respectively, as shown in Table 1.
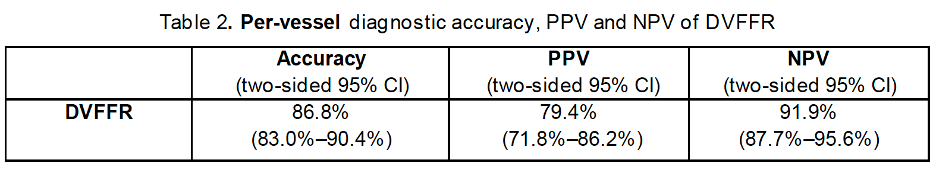
The observed diagnostic accuracy, PPV (positive predictive value) and NPV (negative predictive value) of DVFFR were 86.8% (95% CI: 83.0%–90.4%), 79.4% (95% CI: 71.8%–86.2%) and 91.9% (95% CI: 87.7%–95.6%), respectively. The results are summarized in Table 2.
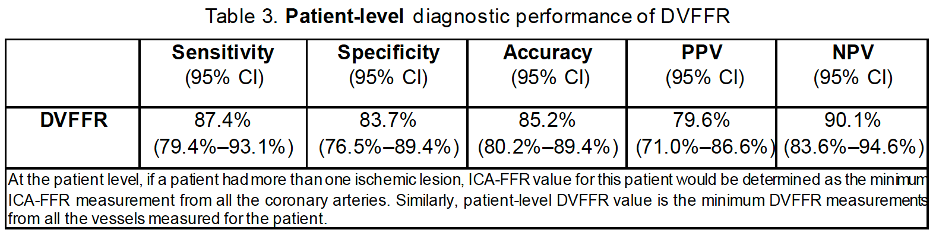
At the patient level, the observed sensitivity, specificity, accuracy, PPV, and NPV were 87.4% (95% CI: 79.4%–93.1%), 83.7% (95% CI: 76.5%–89.4%), 85.2% (95% CI: 80.2%–89.4%), 79.6% (95% CI: 71.0%–86.6%), and 90.1% (95% CI: 83.6%–94.6%), respectively, as shown in Table 3.
DEEPVESSEL FFR
Classification Name: Coronary Vascular Physiologic Simulation Software
Regulatory Class: Class II
Product Code: PJA
Predicate Device: HEARTFLOW, INC.’s FFRCT V2.0 (K161772)
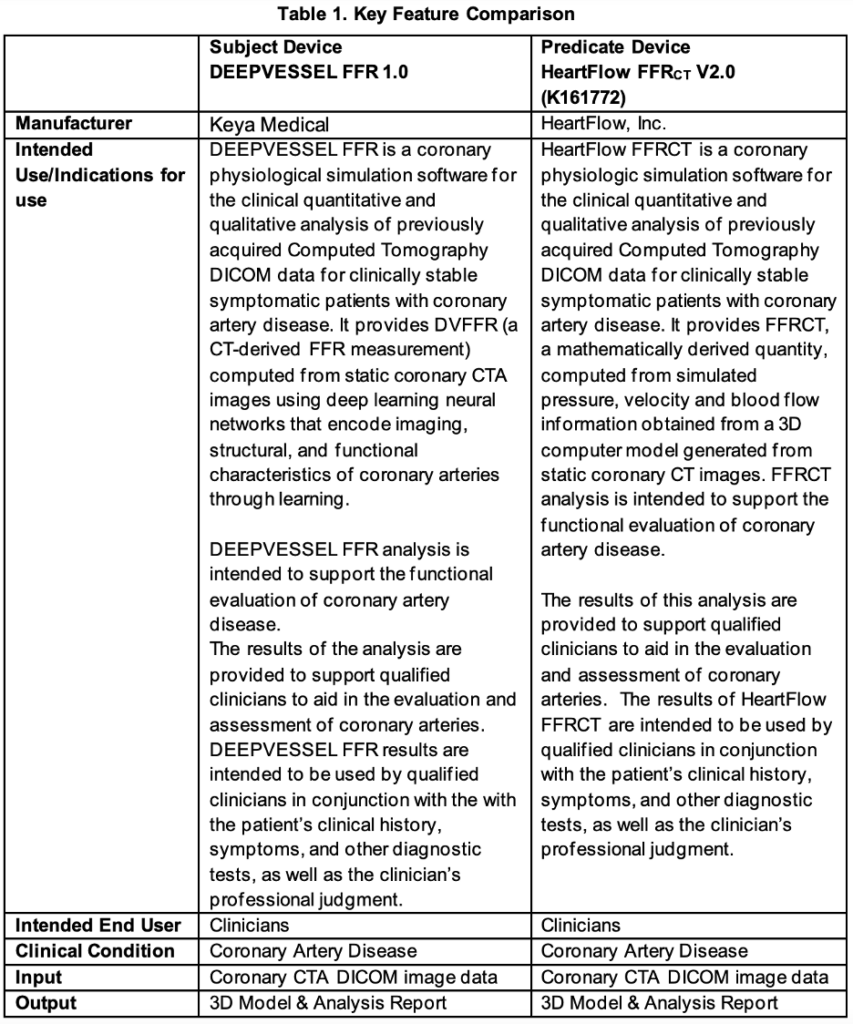
DEVICE DESCRIPTION AND / INDICATIONS FOR USE
DEEPVESSEL FFR is a coronary physiological simulation software for the clinical quantitative and qualitative analysis of previously acquired Computed Tomography DICOM data for clinically stable symptomatic patients with coronary artery disease. It estimates FFR values from static coronary CTA images with extracted coronary tree structures using deep learning neural networks.
DEEPVESSEL FFR analysis is intended to support the functional evaluation of CAD.
The software processes these images semi-automatically, and it generates a 3D model of the coronary artery tree and computes DVFFR (CT-derived FFR) values. Qualified image analysts interact with the software by providing manual edits to the 3D coronary artery tree segmentations when needed and oversees outputs along the processing steps. DVFFR analysis results are sent electronically to the physicians via a third-party service portal application. The results of the analysis are provided to support qualified clinicians to aid in the evaluation and assessment of coronary arteries.
DEEPVESSEL FFR results are intended to be used by qualified clinicians in conjunction with the with the patient’s clinical history, symptoms, and other diagnostic tests, as well as the clinician’s professional judgment.
DVFFR software is independent of imaging equipment, imaging protocols and equipment vendors; the clinical validation study report includes the specific imaging scanner types and imaging acquisition parameters used in the clinical validation of the product.
SUMMARY OF TECHNOLOGICAL CHARACTERISTICS
DEEPVESSEL FFR is a software medical device that is designed to be used by qualified image analysts (trained and certified professionals) to analyze coronary CTA images of clinically stable
symptomatic patients with CAD. It calculates CT-derived FFR values from static coronary CTA images using deep learning neural networks. DEEPVESSEL FFR analysis is intended to support the functional evaluation for clinical stable CAD patients.
The software generates the DVFFR analysis results in two main steps. The first step generates a 3D coronary artery tree model from the CTA image automatically using deep learning-based segmentation algorithms. Manual corrections of the segmentation results are allowed when necessary to confirm the accuracy of the 3D coronary artery tree segmentation. In the second step, the deep learning framework consists of a multi-layer perceptron network (MLP) and a bidirectional multi-layer recursive neural network (BRNN), which utilize the segmentation results and the CTA image, to estimate semi-continuous FFR values along the coronary artery centerlines. The output of the analysis is a PDF report with detailed DVFFR assessment and branch-by-branch visualizations, along with a 3D DVFFR tree model where the DVFFR values are mapped on top of the surface model.
DEEPVESSEL FFR and the predicate device have similar technological characteristics, utilizing computational models to generate CT-derived FFR value for interpretation.
OTHER PERFORMANCE DATA
The following testing have been conducted to demonstrate the substantial equivalence:
Software: Software verification and validation activities were performed according to written
procedures and FDA Guidance document Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices. Verification and validation testing confirmed that the predefined acceptance criteria have been fulfilled.
Human Factors Evaluations: Two human factors studies were conducted in accordance with FDA’s Guidance Applying Human Factors and Usability Engineering to Medical Devices, 2016. The studies evaluated the critical tasks associated with use of the device for both physicians and analysts. The findings from the study demonstrated that all critical tasks were completed without use error or difficulty. These study results concluded that the DEEPVESSEL reports are safe and effective for the physician user group for the intended uses and use environment. Additionally, the analyst user group were able to safety and accurately use the DVFFR software and generate reports.
Reproducibility/Repeatability Evaluations: Reproducibility & Repeatability (R&R) testing was performed on a group of CT scans with diverse disease conditions and image qualities to evaluate the variation of repeated analyses of DEEPVESSEL FFR with different image analysts (reproducibility) at different days with a washout-period in between to avoid memory effects (repeatability). Testing results met the pre-specified variability metric threshold and thus demonstrated acceptable performance.
____________________________________________________________________________
SOURCES
- Keya Medical, and Medical University of South Carolina. 2022. “Assessment of the DiAgnostic Performance of DeepVessel FFR in SuspecTed Coronary Artery Disease.” Clinicaltrials.gov. April 20, 2022. https://clinicaltrials.gov/ct2/show/NCT04828590.
- “Food and Drug Administration,” accessed May 7, 2023, https://www.accessdata.fda.gov/cdrh_docs/pdf21/K213657.pdf.
Learn more about DEEPVESSEL FFR
We are actively looking for clinical partners in the United States and European Union.
About Keya Medical
Keya Medical is an international medical technology company developing deep learning-based medical devices for disease diagnosis and treatment. The company is committed to creating solutions that deliver clinical value at all stages in the patient care process, covering specialties including cardiology, neurology, pulmonology, pathology, and surgery. Keya Medical has four centers of excellence in Beijing, Shanghai, Shenzhen, and Seattle. Follow us on LinkedIn, Twitter, and Facebook.
Media Contact
contact@keyamedna.com

Recent Comments