TARGET Randomized Trial
On-Site Deep Learning-Based FFRCT
Safe, Feasible, Informative Strategy in the CAD Management
The on-site FFRCT testing modality, DEEPVESSEL® FFR; Keya Medical Technology, received CE Mark in 2018, was approved by China’s National Medical Products Administration in 2020, and received 510(k) clearance from the US Food and Drug Administration in 2022.
TARGET is the first randomized trial to assess on-site machine learning-based CT-FFR strategy
Clinical Benefits
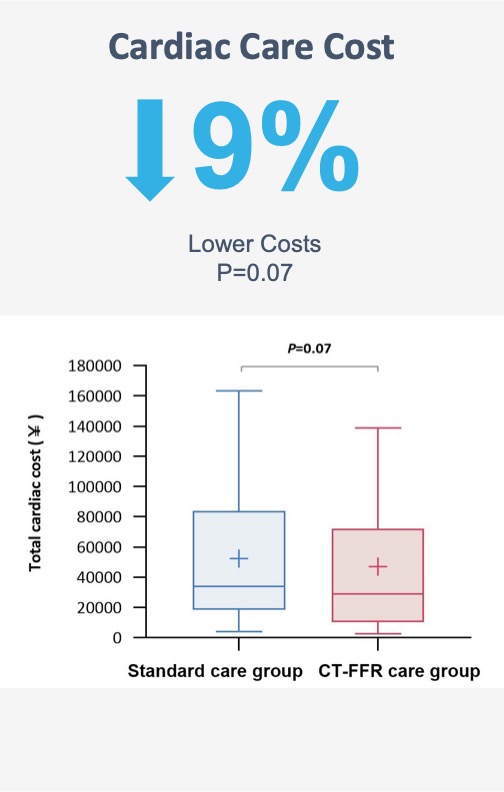
Improve clinical decision-making, patient management, and lower costs
- Feasible, safe, and effective in patients with stable CAD and an intermediate stenosis on CCTA.
- Improves accuracy of identifying patients who are more likely to have obstructive disease suitable for revascularization.
An on-site machine-learning based CT-FFR strategy is feasible, safe, and effective
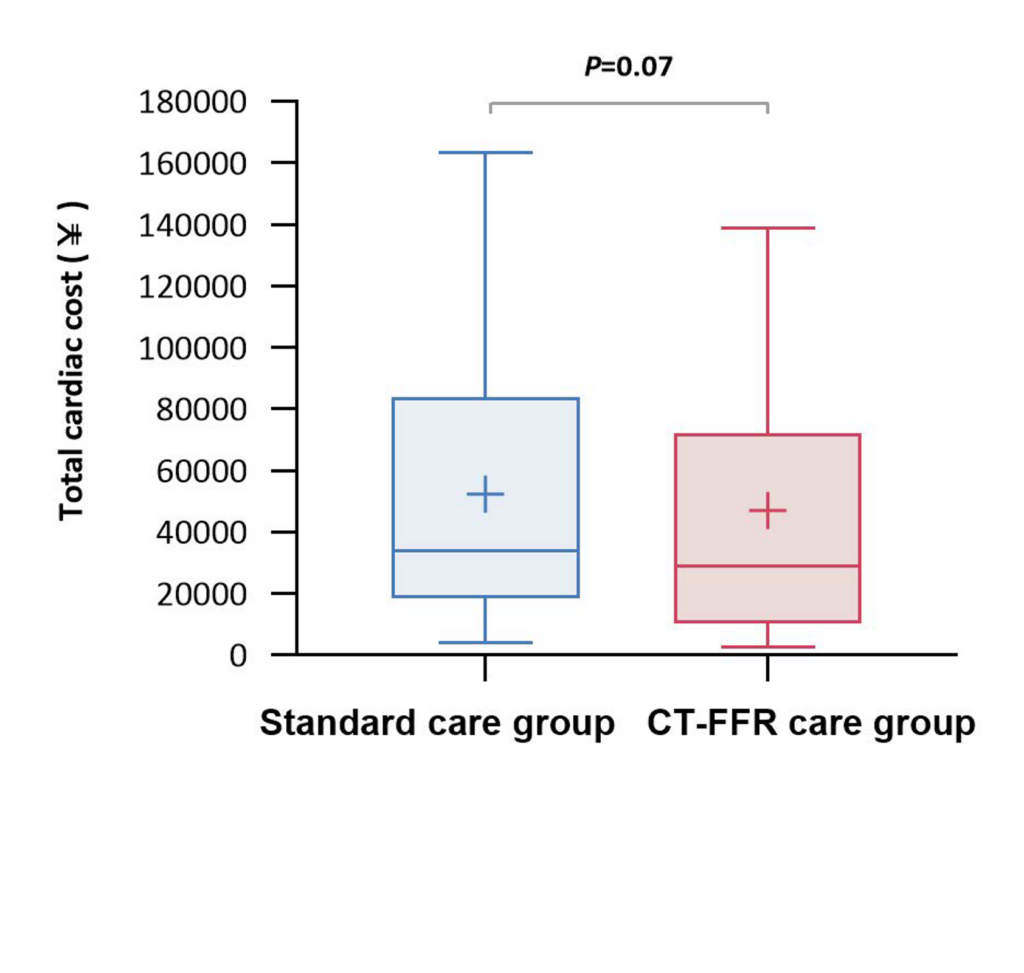
An on-site approach does have the potential to be cheaper and to more rapidly return results
Key Findings
CT-FFR Care Group and Standard Care Group
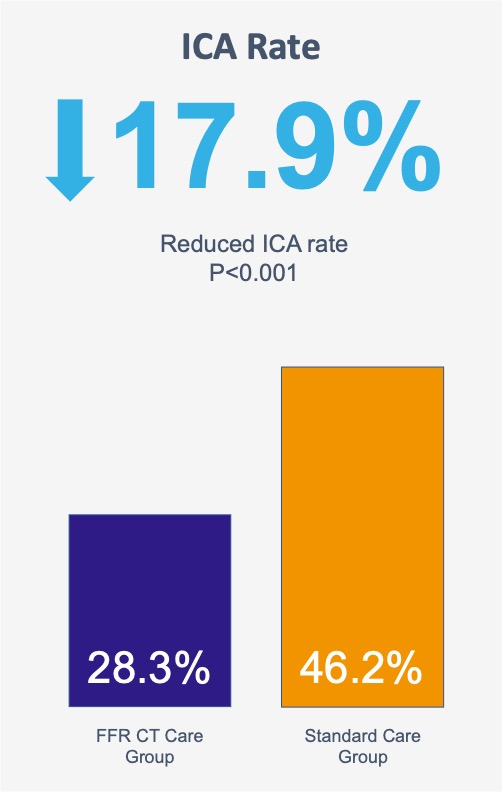
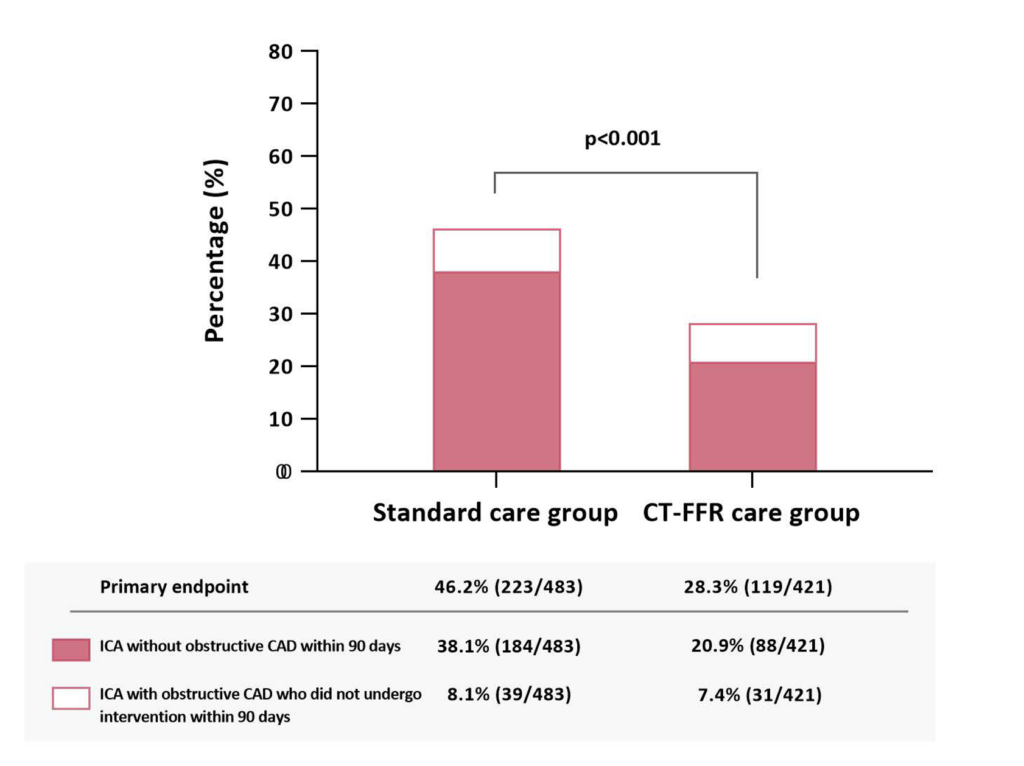
Patients undergoing invasive coronary angiography without obstructive CAD or with obstructive disease not undergoing intervention was significantly reduced in the CT-FFR care group (28.3% [119/421] vs. 46.2% [223/483]).
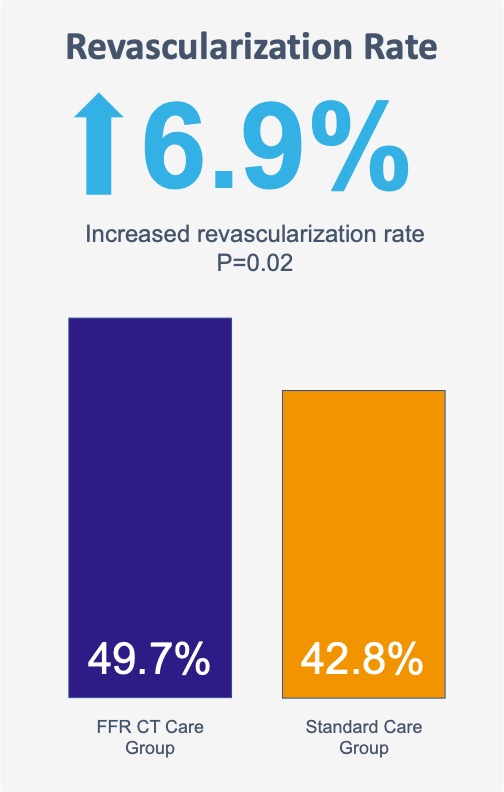
Overall more patients underwent revascularization in the CT-FFR care group than in the standard care group (49.7% [302/608] vs. 42.8% [260/608])*.
*The higher revascularization rate can be due to: the intermediate-to-high risk characteristic of the enrolled patients compared to the PROMISE trial [8]; the rate of 71.7% of performed FFR-CT, compared to 16% in the FORECAST trial [9].
TARGET Trial Design
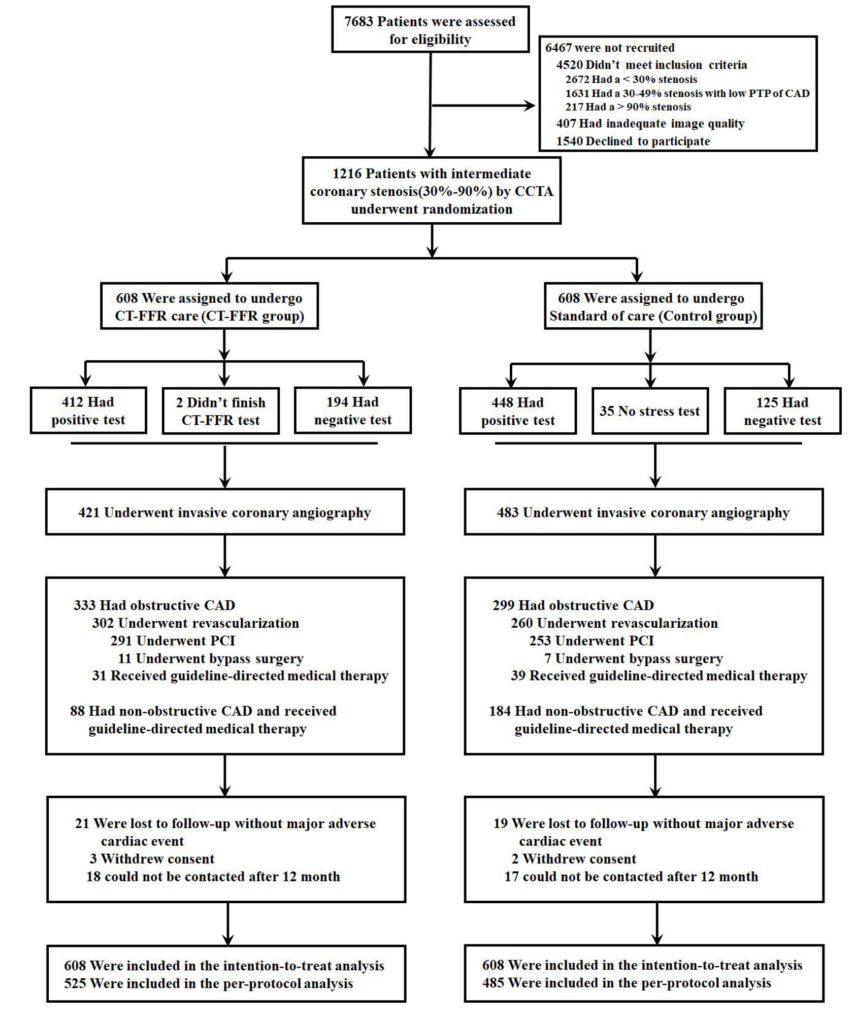
Patients with stable coronary artery disease and an intermediate stenosis of 30% to 90% on CCTA. On-site CT-FFR calculated from CCTA for all major epicardial coronary arteries (≥ 2.5mm).
6
Medical Centers in China
1216
Patients
1:1
Randomized
Prospective
Multicenter
Primary Endpoint
The proportion of patients undergoing invasive coronary angiography without obstructive coronary artery disease or with obstructive disease who did not undergo intervention within 90 days.
Secondary Endpoint
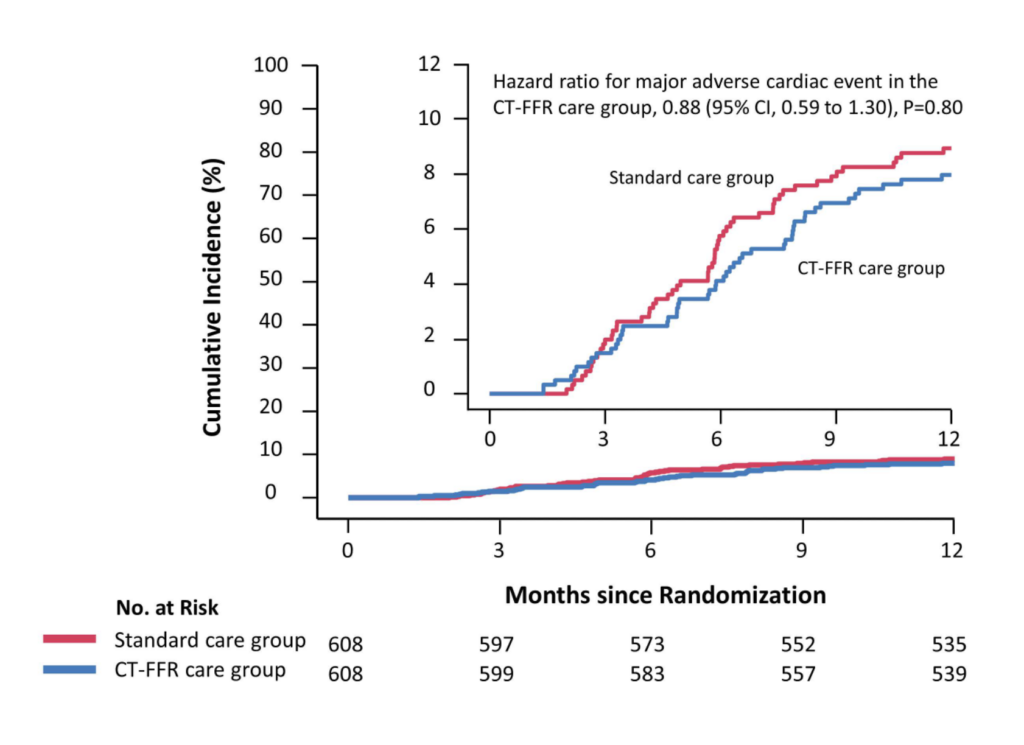
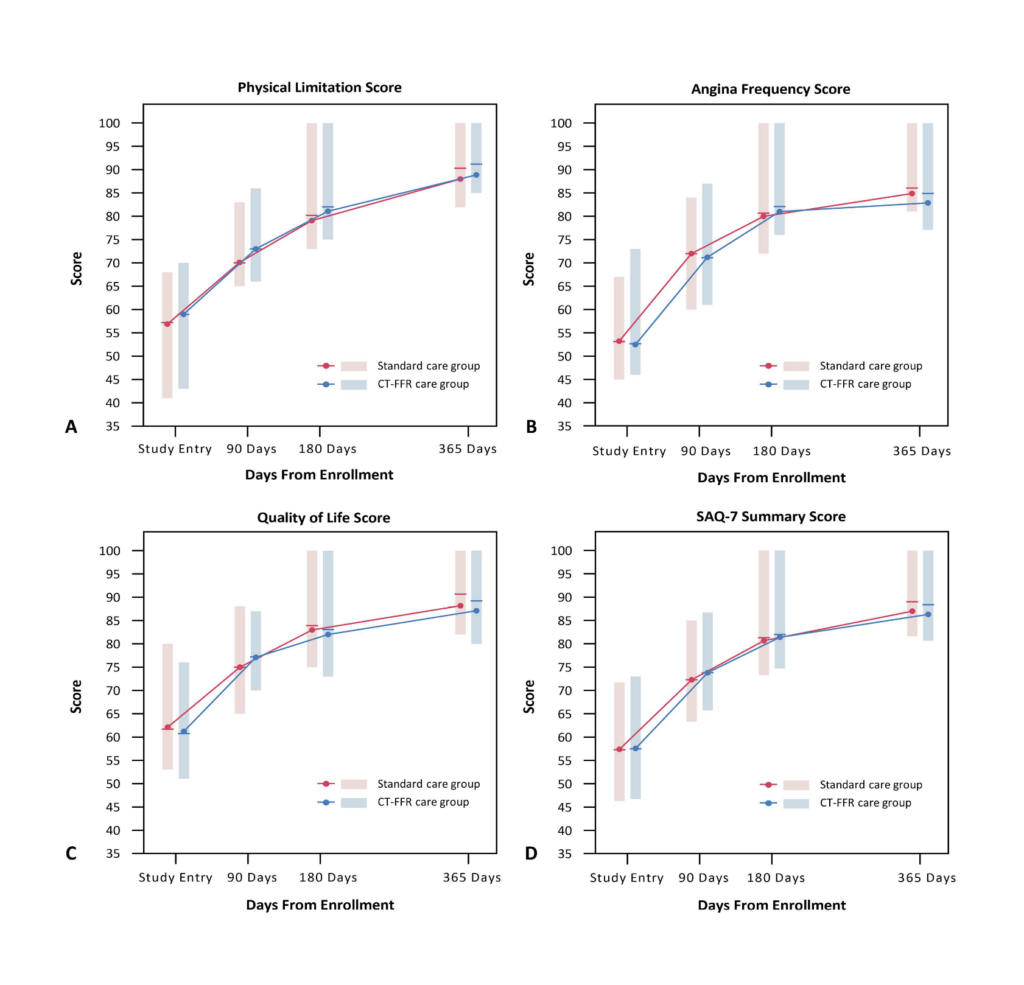
Major adverse cardiovascular events (MACE), quality of life, symptoms of angina,
and medical expenditure at 1 year.
Learn more about DEEPVESSEL FFR
We are actively looking for clinical partners in the United States and European Union.
REFERENCES
- Yang J, Shan D, Dong M, Wang Z, Ma X, Hu X, Zeng HChen Y. The effect of on-site CT-derived fractional flow reserve on the management of decision making for patients with stable chest pain (TARGET trial): objective, rationale, and design. Trials. 2020;21:728.
- On-Site Computed Tomography-Derived Fractional Flow Reserve to Guide the Management of Patients with Stable Coronary Artery Disease: The TARGET Randomized Trial. AHA Journals. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.123.063996
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
- Coenen A, Kim YH, Kruk M, Tesche C, De Geer J, Kurata A, Lubbers ML, Daemen J, Itu L, Rapaka S, et al. Diagnostic Accuracy of a Machine-Learning Approach to Coronary Computed Tomographic Angiography-Based Fractional Flow Reserve: Result From the MACHINE Consortium. Circ Cardiovasc Imaging. 2018;11:e007217.
- Liu X, Mo X, Zhang H, Yang G, Shi CHau WK. A 2-year investigation of the impact of the computed tomography-derived fractional flow reserve calculated using a deep learning algorithm on routine decision-making for coronary artery disease management. Eur Radiol. 2021;31:7039-7046.
- Li Y, Qiu H, Hou Z, Zheng J, Li J, Yin YGao R. Additional value of deep learning computed tomographic angiography-based fractional flow reserve in detecting coronary stenosis and predicting outcomes. Acta Radiol. 2022;63:133-140.
- Yang J, Shan D, Dong M, Wang Z, Ma X, Hu X, Zeng HChen Y. The effect of on-site CT-derived fractional flow reserve on the management of decision making for patients with stable chest pain (TARGET trial): objective, rationale, and design. Trials. 2020;21:728.
- Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291-300.
- Curzen N, Nicholas Z, Stuart B, Wilding S, Hill K, Shambrook J, Eminton Z, Ball D, Barrett C, Johnson L, et al. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: the FORECAST randomized trial. Eur Heart J. 2021;42:3844-3852.
- Yang J, Shan D, Wang X, et al. On-site computed tomography-derived fractional flow reserve to guide the management of patients with stable coronary artery disease: the TARGET randomized trial. Circulation. 2023;Epub ahead of print.
- Cox, Caitlin E. “On-Site Machine Learning-Based FFRCT Feasible, Informative: Target.” TCTMD.com. TCTMD.com, March 4, 2023. https://www.tctmd.com/news/site-machine-learning-based-ffrct-feasible-informative-target.
- The TARGET trial was sponsored by grants from the National Key R&D Program of China and Beijing NOVA Program.
- Dr. Chen reports consultant fees/honoraria from Cordis and Medtronic.
- Douglas reports research grants to her institution from HeartFlow and funding from the National Institutes of Health for the PROMISE trial.