Keya Medical’s DEEPVESSEL FFR offers a non-invasive, accurate, and efficient solution for assessing coronary artery function. The product’s journey from inception to global clearances reflects a commitment to advancing medical technology and improving patient outcomes in cardiovascular health.
In the fast-developing landscape of medical technology, Keya Medical emerges as a pioneering force in AI medical device technologies for cardiovascular care with its flagship product, DEEPVESSEL FFR.
On November 8 Mary-Pierre Waiss, Vice President of Market Development presented Keya Medical AI-based CT FFR technology at the 7th “Technology Diplomats Innovation Resources Matchmaking Action” conference in Beijing, China. The event was hosted by the Beijing International Cooperation Center of Science and Technology. Since its inception in 2016, this annual event has successfully integrated scientific and technological innovation resources from over 30 countries, creating a powerful international exchange platform. With a focus on “contributing to future development through technology cooperation,” the meeting drives international collaboration in science and technology.
Keya Medical Overview
Keya Medical, an international medical technology company established in 2016, has been at the forefront of developing deep learning-based medical devices across various specialties, including cardiology, neurology, pulmonology, pathology, and surgery. With offices in Seattle, Shanghai, Shenzhen, and Beijing, the company has collaborated with hospitals and key opinion leaders worldwide.
DEEPVESSEL FFR: AI Innovation in Cardiovascular Assessment
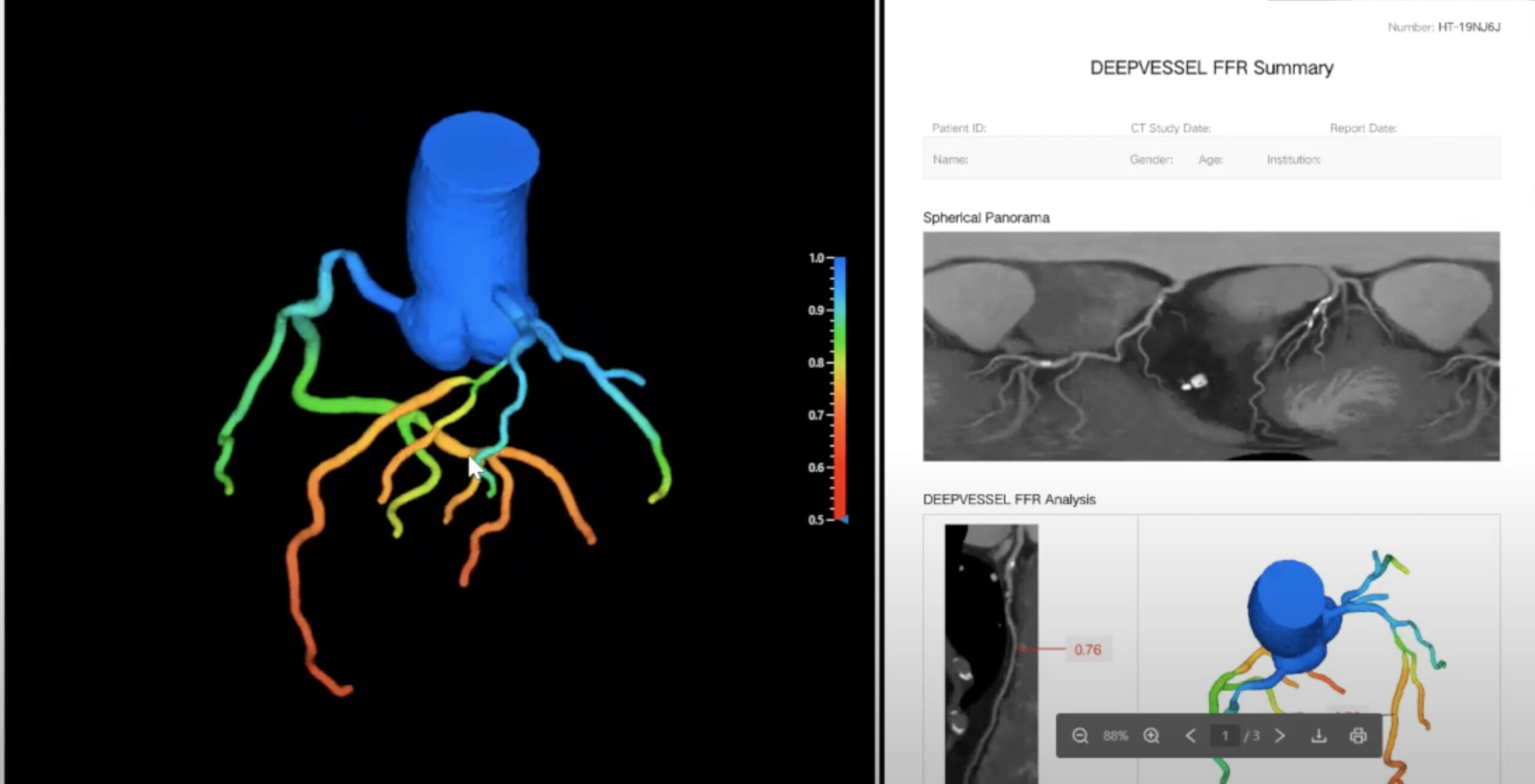
DEEPVESSEL FFR stands out as a non-invasive coronary artery functional assessment tool that utilizes deep-learning neural networks. The product offers a non-invasive physiological functional assessment of coronary arteries derived from CT angiography (CTA). This innovative approach generates a 3D interactive model and a PDF report, providing physicians with quick and accessible results electronically.
Product Milestones
The journey of DEEPVESSEL FFR reflects Keya Medical’s commitment to excellence. The company received CE Mark approval in 2018, Chinese FDA clearance in 2020, and US FDA clearance in 2022. These clearances were achieved through extensive research and validation, including the multicenter ADAPT study.
Clinical Significance of CT FFR
CT FFR brings significant clinical value by improving the triage of patients with chest pain and suspected CAD. Traditionally, patients underwent invasive angiography, but with the rise of CT scanners, cardiac CTA became the initial assessment. DEEPVESSEL FFR further refines this process by focusing on patients with stenosis between 30% and 90%, enhancing the triage and avoiding unnecessary invasive tests.
Professional Recognition and Guidelines
Professional societies, including the American Heart Association and the American College of Cardiology, have recognized the importance of CT FFR. Guidelines emphasize the significance of CTA and FFR as frontline tests for patients with chest pain. Numerous trials and studies, both prospective and retrospective, underscore the importance of CT-based FFR analysis in ongoing patient care.
Advantages of DEEPVESSEL FFR
The unique advantages of DEEPVESSEL FFR include its non-invasive nature, high accuracy, efficient evaluation of FFR calculations, and quick accessibility of results on various devices. The product’s use of deep learning neural networks enhances computational efficiency and performance with increasing data.
Live Demonstration
The demonstration of DEEPVESSEL FFR showcases its interactive coronary artery tree. Physicians can rotate, enlarge, and interrogate the vessels at any point, obtaining crucial information on fractional flow reserve percentages and vessel diameters. The accompanying PDF reports offer a comprehensive overview, aiding in decision-making for patient care.
Media Contact
contact@keyamedna.com
Learn more about DEEPVESSEL FFR
We are actively looking for clinical partners in the United States and European Union.
