
Advancements in AI-based CT-FFR Analysis with DEEPVESSEL FFR: A Comprehensive Research Overview
Keya Medical’s AI CT-FFR Analysis: focus on its research and publications journey since DEEPVESSEL FFR FDA-clearance.

Keya Medical’s AI CT-FFR Analysis: focus on its research and publications journey since DEEPVESSEL FFR FDA-clearance.

Visit Keya Medical’s booth at SCCT Global 2024 in Budapest, January 16-19.
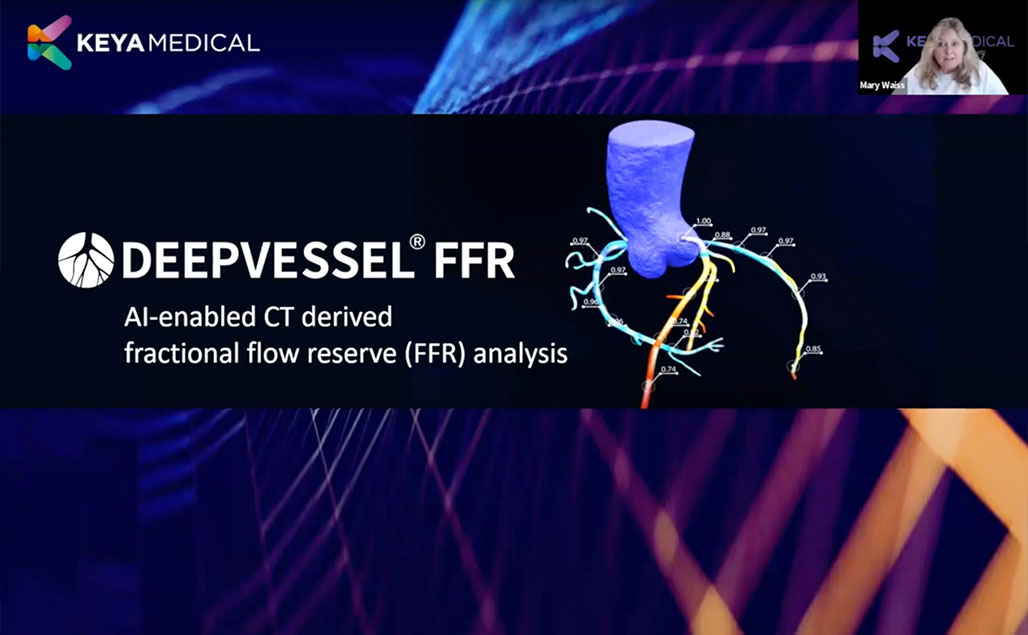

In the dynamic landscape of medical innovation, SCCT 2023 served as an exceptional platform for exchanging cutting-edge insights and breakthroughs in cardiovascular technology.

Keya Medical US celebrates the achievements of the team in China and their commitment to the commercialization of DEEPVESSEL FFR.
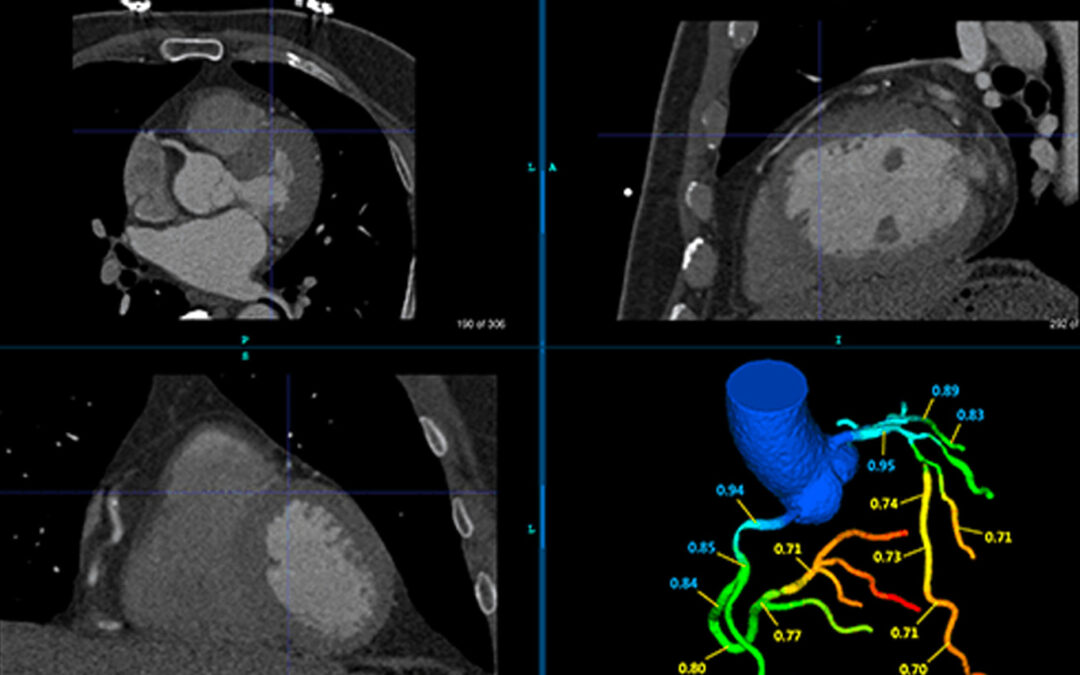
Seattle, WA– April 1, 2022– Keya Medical announced that DEEPVESSEL FFR (DVFFR), has been cleared by U.S. Food and Drug Administration (FDA).
Recent Comments