
by KeyaMedical | Feb 25, 2021 | All News, Press
Keya Medical’s President of R&D, Dr. Kunlin Cao, has been recognized on the 2021 Forbes China Up-and-Coming Businesswomen list. The list highlights 20 accomplished women who have each played a significant role in shaping China’s business landscape.
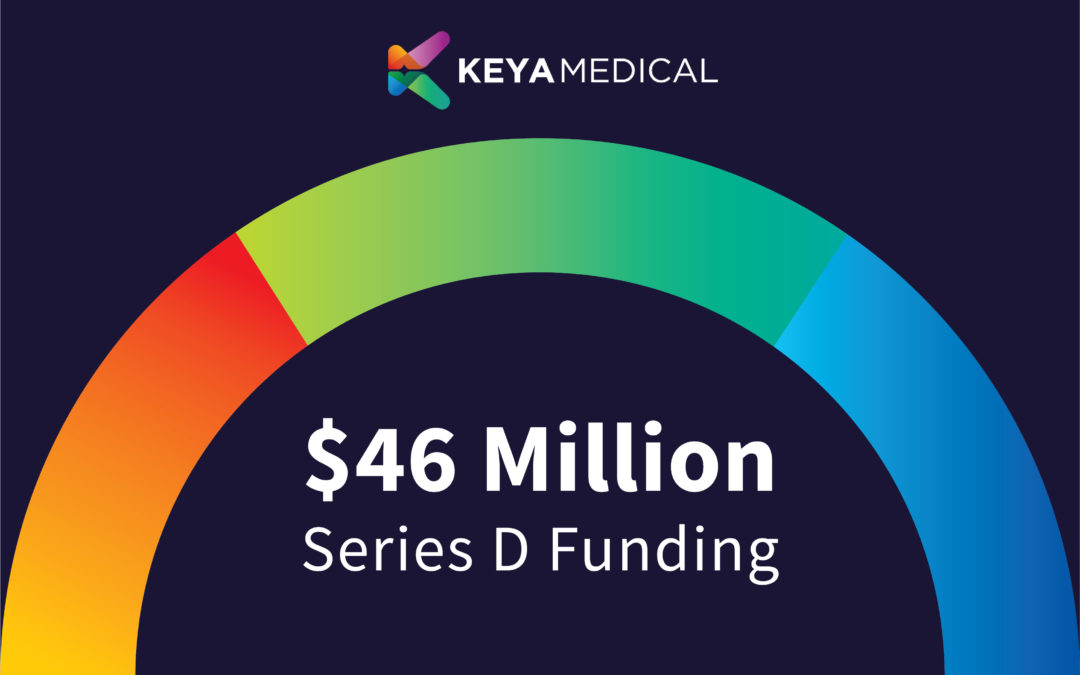
by KeyaMedical | Jan 14, 2021 | All News, Investors, Press
Keya Medical has raised $46 million in Series D funding. This funding round was led by new and existing investors, including CICC Capital, Shanghai Artificial Intelligence Investment Fund, Qianhai Gaozu Asset Management Fund, and IN CAPITAL.

by KeyaMedical | Dec 12, 2020 | All News, Press
Keya Medical, a leading medical AI technology company, was recognized as one of the top 50 Global Healthcare Technology Companies at the 2020 World Innovators Meet (WIM) conference held By Equal Ocean in Beijing from December 9-11.

by KeyaMedical | Nov 12, 2020 | All News, Investors, Press
Keya Medical announced that it has completed a $30 Million Series C Funding Round led by IDG Capital, with participation from investors Alwin Capital, Source Code Capital, and Tasly Group.

by KeyaMedical | Oct 19, 2020 | Press
On Oct. 17 and 18, the Chengdu Science and Technology Bureau hosted the 2020 DEMO China Innovation Finals, bringing together leading companies across the country.

by KeyaMedical | Sep 18, 2020 | Press
On Sept. 18, Keya Medical was recognized as the winner of China’s Most Promising Company award because of its leading R&D team and innovative cardiovascular product line.







Recent Comments