
The future of AI-driven, noninvasive CT FFR technology-DeepVessel FFR at 2022 cardiology events in China
Keya Medical US celebrates the achievements of the team in China and their commitment to the commercialization of DEEPVESSEL FFR.

Keya Medical US celebrates the achievements of the team in China and their commitment to the commercialization of DEEPVESSEL FFR.
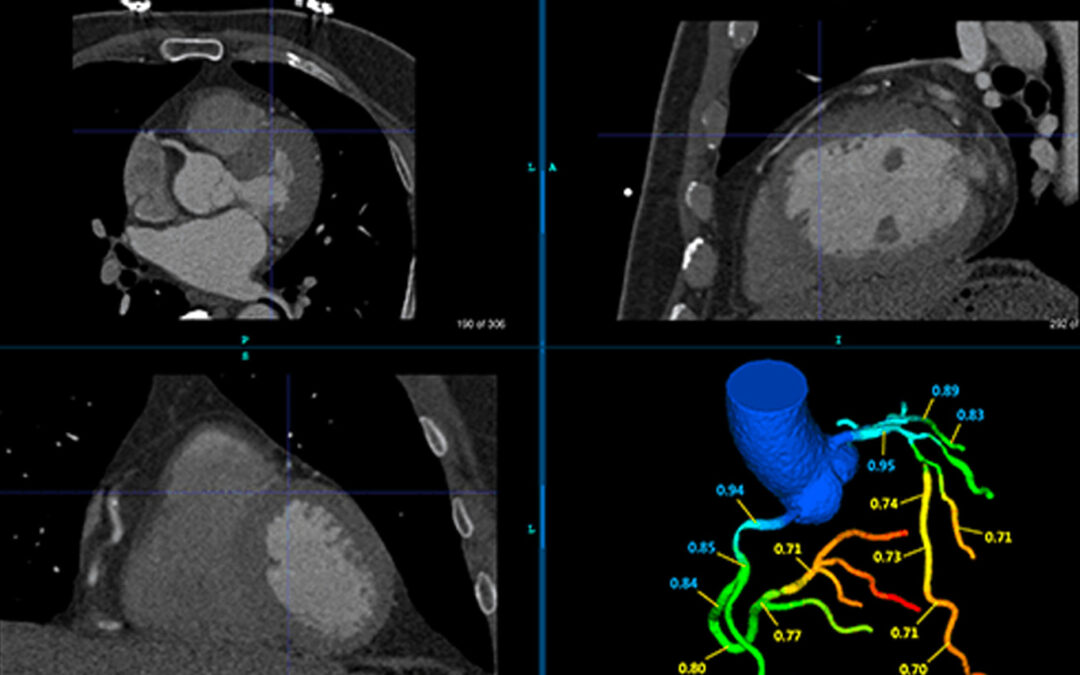
Seattle, WA– April 1, 2022– Keya Medical announced that DEEPVESSEL FFR (DVFFR), has been cleared by U.S. Food and Drug Administration (FDA).
Recent Comments